推进新冠肺炎疫苗公平分配
Advancing Research and Planning for the Equitable Distribution of a Covid-19 Vaccine

By Katherine Bliss, Senior Fellow, Global Health Policy Center
With more than 12 million confirmed cases of Covid-19 worldwide, and with more than 550,000 deaths attributed to complications from infection with the novel coronavirus, identifying and deploying new diagnostics, treatments, and ways to prevent infection remains critical. For many experts, developing and globally distributing a safe and effective vaccine offers the greatest hope of returning to normal economic and social life. But with vaccine research centered in high-income countries, and with confirmed cases of Covid-19 increasing rapidly in lower- and middle-income countries, it is important to lay out plans now for ensuring equitable access to, and effective distribution of, a vaccine, or vaccines, once available.
At a Saturday, June 27, pledging event, “Global Goal: Unite for Our Future,” hosted by the nongovernmental organization Global Citizen in cooperation with the European Commission, donor governments, foundations, and corporate entities pledged at least $1.3 billion in grants, loans, and guarantees to support the distribution of Covid-19 tests, treatments, and vaccines and ensure that needed tools reach lower- and middle-income countries. The event also generated funds to support the Access to Covid Tools (ACT) Accelerator, the entity launched in April by a consortium of global health organizations, including the European Commission; the World Health Organization (WHO); Gavi, the Vaccine Alliance; the Coalition for Epidemic Preparedness Innovations (CEPI); the Global Fund to Fight AIDS, Malaria, and Tuberculosis; the Wellcome Trust; and the Bill & Melinda Gates Foundation, among others, to support the development of essential products to respond to the pandemic.
Since the emergence of Covid-19, Gavi has played a prominent role in guiding global conversations regarding the relevance of strong immunization and health systems to international response efforts. When the WHO declared the outbreak of the novel coronavirus, known as Covid-19, to be a pandemic in early March, the majority of cases had been reported in China and Europe, with the number of confirmed infections just starting to increase in the United States. But as the pandemic has intensified over the past few months, with the outbreak expanding to Russia, South America, India, and sub-Saharan Africa, 70 of the 73 Gavi-eligible countries have confirmed cases. Acknowledging that the lowest-income countries would have few resources to help prepare for an inevitable wave of Covid-19, in March the Gavi Board authorized countries that had received Gavi support for health system and immunization system strengthening activities to apply to redirect up to 10 percent of those funds for Covid-19 response. As of mid-June, at least 38 countries had applied to reprogram health system strengthening funds for activities, including infection prevention and control, disease surveillance, and enhancing laboratory testing capabilities.
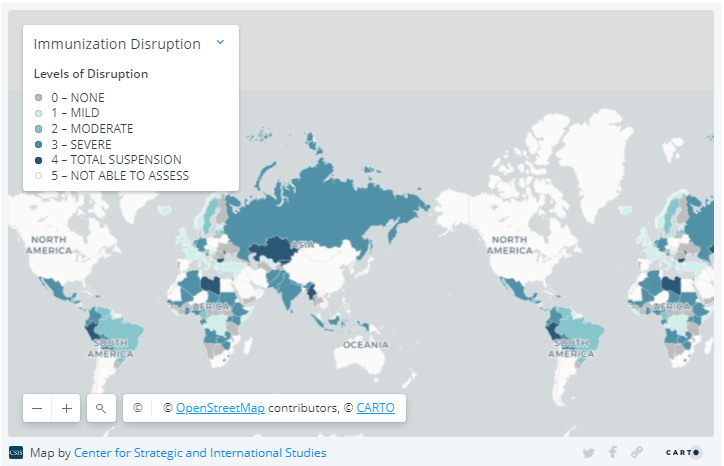
Source: World Health Organization.
The Covid-19 response has shifted attention away from all routine health services, but immunization services have been particularly hard hit. A mid-April poll coordinated by the WHO, UNICEF, Gavi, and the Sabin Vaccine Institute’s Boost Initiative and distributed through local partners revealed that routine immunization programs had been disrupted in nearly two-thirds of countries surveyed. In several Gavi countries, plans to introduce new vaccines have been delayed as well. Acknowledging that Covid-19-related activities are diverting health workers from delivering vaccines, that restrictions on transportation and air travel may have limited the supply chain for vaccines and vaccine supplies, and that families may be hesitant to take children to health clinics for immunizations out of concern they may be exposed to the virus while at the facility, Gavi has joined the WHO and UNICEF in encouraging countries to maintain their routine immunization programs if at all possible, or to restart them as soon as feasible. The costs of providing immunization services during the Covid-19 outbreak are likely to rise, given the need to install additional equipment such as handwashing stations, provide staff with personal protective equipment (PPE), and remodel waiting areas to accommodate new space requirements due to the social distancing dictates of Covid-19 prevention. Nevertheless, experts warn that these costs will be less than those associated with treating outbreaks of vaccine-preventable disease and waging expensive emergency catch-up campaigns in a context of accelerating Covid-19 transmission.
In early June, at the Global Vaccine Summit 2020, hosted by the United Kingdom and held virtually, Gavi secured $8.8 billion in pledges for its 2021-2025 work plan. The event also served to introduce two new initiatives focused on Covid-19, the Covax-19 Global Vaccine Access Facility (COVAX Facility) and the Gavi Advance Market Commitment for Covid-19 Vaccines, to ensure availability of a new Covid-19 vaccine (or vaccines), once available, to populations in need, including those in the world’s poorest countries. Governments making direct pledges to Gavi included longtime supporters, such as the United Kingdom, Norway, and the United States, along with Canada, Japan, and Spain. The group making pledges also included first-time donors Bhutan, Burkina Faso, Cameroon, Finland, Greece, New Zealand, Portugal, and Uganda.
Some countries designated support for the International Financing Facility for Immunizations (IFFIm), which issues vaccine bonds for use by Gavi via multiyear commitments and transfers of funds to the facility at set intervals. This mechanism for providing long-term support to Gavi has been a choice of some middle-income countries, such as Brazil and South Africa, because it allows them to spread out funding commitments over a 20-year period. The United Kingdom, France, Italy, Australia, Norway, Spain, the Netherlands, and Sweden have made contributions to IFFIm in the past as well. This year, Norway pledged approximately $200 million to IFFIm to pass funding through Gavi to CEPI, which is developing a number of different Covid-19 vaccines and is based in Oslo. Italy, the Netherlands, and Spain also renewed support for IFFIm in 2020.
At the June 4 summit, the ACT Accelerator launched the Covax Facility to promote equitable access to a Covid-19 vaccine, or vaccines, once available. Co-led by Gavi, the WHO, and CEPI, the Facility enables countries to signal in advance their intent to purchase a quantity of doses of vaccine, once licensed and pre-qualified. Through the Facility, high-income countries can self-finance these advance purchases, and donor governments can provide funds to enable lower-and middle-income countries to arrange for purchases.
The Advance Market Commitment (AMC) is a key element of the Facility and is meant to incentivize manufacturers to expand production capacities and make doses of an approved Covid-19 vaccine available at low cost for Gavi countries. At the summit, the Gavi AMC secured more than $500 million of the $2 billion the Alliance estimates will be necessary to cover initial costs of vaccine guarantees and deliver the first doses to health workers and other vulnerable populations in Gavi-eligible countries. However, the overall cost to deliver a new vaccine to populations in Gavi countries is likely to be closer to $16 billion, or higher.
The Covid-19 AMC approach draws on the lessons from a 2009 AMC that made a new vaccine for pneumococcal disease, a leading cause of pneumonia, available to eligible countries with Gavi support. More recently, Gavi oversaw an AMC to make the vaccine that prevents infection with Ebola virus available to eligible countries. Countries choosing to participate in the Covid-19 AMC provide funds and make a commitment to purchase a pre-defined number of vaccine doses at a specified low price, and donors can also contribute funds to ensure doses for populations in lower- and middle-income countries. Funds generated through the new Covid-19 AMC will support the expansion of manufacturing capacity for any new approved vaccine. The pharmaceutical company AstraZeneca has already committed to provide 300 million doses of the Covid-19 vaccine it is developing with the University of Oxford. At the June 27 event, Serum Institute of India committed to dedicating 50 percent of its production capacity to making a Covid-19 vaccine available to low- and middle-income countries.
The U.S. government has been a longtime donor to Gavi and also supports global immunization programs through the provision of bilateral development funds and technical assistance activities. At the June 27 pledging event, the United States joined other G7 members, the European Union, the United Nations, and the African Union in expressing support for the ACT Accelerator and indicated it is directing $545 million to Covid-19 relief efforts. Earlier this year, the United States had reaffirmed its support for the Alliance through a multiyear pledge of $1.16 billion, or $290 million per year for four years. But while the U.S. multiyear pledge to Gavi is important as confirmation of continued support for the Alliance, it is essentially flat funding in a period when other donors, recognizing the essential role immunizations play in protecting health security, have increased their contributions, both to support Gavi’s next five years of work, and to support Covid-19 response, in particular.
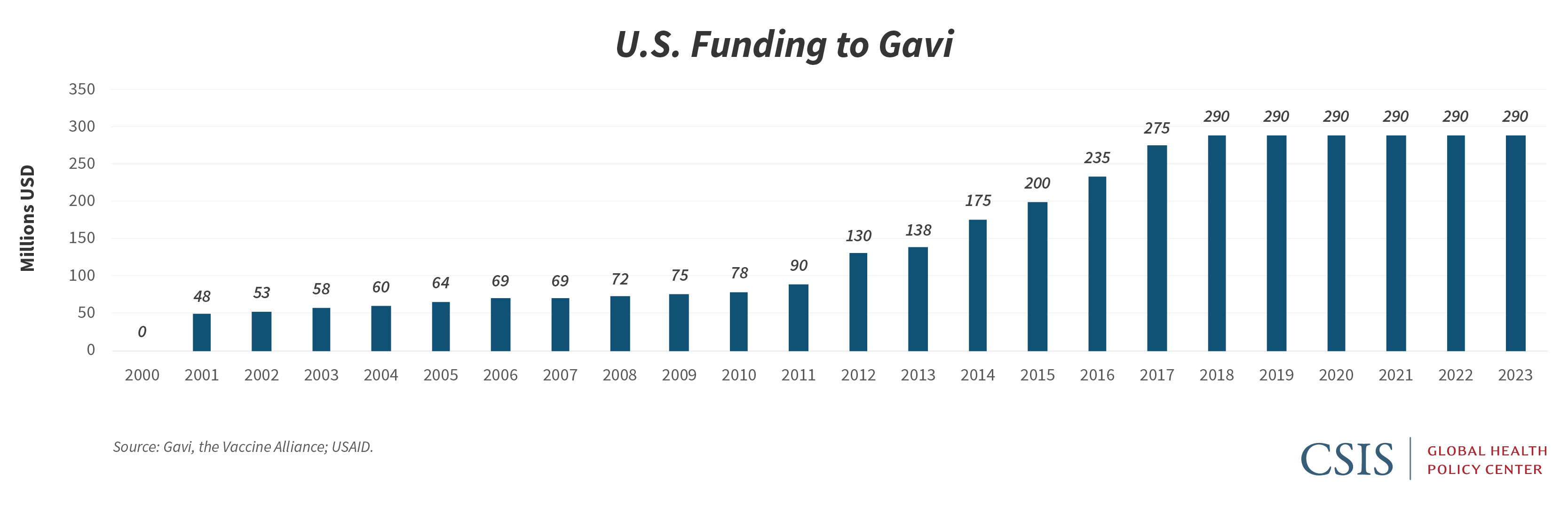
As the United States considers how it can support Gavi and Gavi-eligible countries in responding to the Covid-19 pandemic, it too should consider increasing its direct annual contribution to the Alliance. At the same time, the United States has an opportunity to strengthen bilateral engagement on immunization in countries affected by the outbreak, both through existing support for immunization services and through the current round of global health emergency funding for Covid-19 response. The United States has not contributed to previous Gavi AMC activities, but policymakers should determine the legal framework that could best allow the U.S. government to support the new Gavi AMC for a Covid-19 vaccine, recognizing that such efforts to ensure broad global distribution of a vaccine enhance existing U.S. investments in global health and protect the health of U.S. citizens at home and abroad. The Health and Economic Recovery Omnibus Emergency Solutions Act (HEROES Act) that has passed the House and been placed on the Senate’s calendar for upcoming discussion offers an opportunity for Congress to make an additional commitment to Gavi within the context of a broader set of appropriations related to Covid-19 recovery.
With the Covid-19 outbreak intensifying in lower- and middle-income countries, and with efforts to develop and disseminate a vaccine to prevent infection with Covid-19 proceeding rapidly, now is the time to strengthen the delivery of immunization services globally, improve access to health care at all levels, build trust in public health systems, and ensure the availability of adequate funding and support to ensure the smooth delivery of a Covid-19 vaccine, or vaccines, to all populations in need.
Note on methodology for map on immunization disruption:
Immunization disruption can take the form of disruption to routine immunization services; interruptions in supplies, vaccine stockouts, or vaccine distribution; reduction in health care workers and vaccine personnel; or decreased demand from the public. The WHO classified the levels of disruption as total disruption, total suspension of immunization activities; severe disruption, disruption of routine immunization services; moderate disruption, meaning countries are experiencing at least two of the types of immunization disruption listed above, other than routine immunization services; mild disruption, experiencing only one type of disruption, other than routine immunization services; or no disruption. Please note that the pandemic situation is evolving and some countries that had initially suspended immunization services may have already restarted them.
The data come from a variety of sources gathered from March through May 2020, including poll data. The data are subject to limitations inherent to voluntary self-reporting: self-selection bias, not all countries responded, countries with only one response vis-à-vis countries with many, possibility of fraudulent responses, and not having a sampling frame to make inferences. Furthermore, the information about each country does not represent official reporting from member states to the WHO or UNICEF. Thus, the results presented here need to be interpreted with caution and do not represent in any way a WHO or UNICEF position regarding any country or territory for which one or more replies were received.